Starting the Sweet Starter
An interview with Ian Lowe
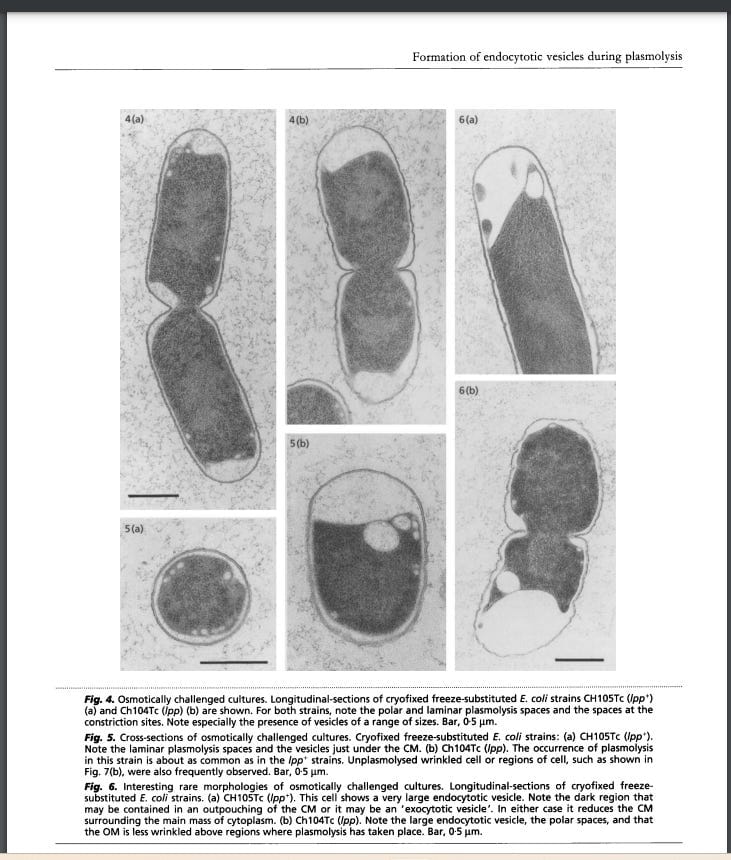
Table of Contents
Ian Lowe is the former owner of the bakery Apiece, in Ravenswood, Tasmania. He’s between bakeries at the moment, but keeps himself very busy consulting others in Australia and beyond on optimizing their production processes, and on sharing his wealth of knowledge on his Instagram account, @apieceofbread.
Ian is the inventor of the “sweet starter” sourdough process, a method for creating a sourdough culture that yields a product with virtually no sourness to it and an extended shelf-life, not unlike the grandi lievitati products such as panettone that inspired it. It involves building a starter with a very low hydration and a precise amount of granulated sugar added to it, which basically decimates the lactic acid bacteria in it, so that the final fermentation is almost exclusively yeast-raised.
I discovered the sweet starter process online, and have made some beautiful breads with it since then, but I noticed that many of the methods recommended by others did not adhere to his original formulation. Wanting to know more, and to hear more about how he came up with the idea and how it works, I reached out to Ian to ask if he’d be willing to discuss it with me here.
Ian has a reputation for being something of a curmudgeon online, with limited tolerance for those who position themselves as authorities on bread baking without having the goods to back it up. As he says below, he is autistic, which explains his sometimes brusque manner. But if you follow his Instagram presence closely, it’s obvious that he’s actually a generous and patient person, willing to answer nearly any and all questions posed to him, and he clearly has the goods himself. And he offers his knowledge freely, wanting nothing more than to have accurate information out in the world. My interview with him is a perfect example of this: It took place over email, over the course of several months (and dozens of emails), and he never once seemed to lose patience with my many questions, intelligent or otherwise.
I really love the sweet starter method, and I’m grateful to Ian for taking the time to help me understand it better. I hope the same proves true for some of you.
As seems to be a habit around here, this is another long interview, but once you dig in, it should be clear why it needs to be. After you finish it, be sure to check out the accompanying post I created, which walks you through the sweet starter method in detail should you want to try it out for yourself:

—Andrew
Andrew: How did you come up with the idea for the sweet starter in the first place?
Ian Lowe: I started my first bakery, Apiece, in 2012. I made an impromptu decision to make all leavened products using only sourdough. This was more as a challenge to myself rather than for any lofty, idealistic considerations. There's a Chuck Close saying I quite like: “Get yourself in trouble.” It means, put yourself into intentionally-uncomfortable positions, where nobody can simply provide the answers. The solution you arrive at will be unique and novel to you.
I had little money, starting with only two wood-top benches, a dodgy, inherited four-deck oven, and a cheap spiral mixer. There were few resources, either online or off, for how to make sourdough breakfast pastries that have less than 25% sugar to total flour weight, like croissants or cinnamon rolls. My goal was to make, say, a pain au chocolat or a bombolone with no detectable sourness.
I instinctively knew to look at tradition, specifically Italian grandi lievitati, festive celebration breads like Milan's panettone or Verona's nadalin. Unfortunately, the approach used for these products isn't helpful, as they're much more enriched with sugars and fats and contain far less water. (Below, we'll cover why that's a problem.) What's more, they are labour- and equipment-intensive. I was a one-man band in the beginning. I had no proofer.
I'm autistic. My obsession is food. I also have excellent research skills. (Before dropping out of uni, I wrote papers for a living, even fully researching and writing others' theses or dissertations.)
I'd already begun looking into bread science earnestly, because much of what's written in mainstream baking books didn't jibe with my own personal experience. E.g., it's often taught that warmer, wetter doughs lead to more lactic acid, and colder, stiffer doughs more acetic. I wanted to understand why this wasn't the case, as I never found this to true. (Turns out, like many baking truisms, it's not.)
I came across three Italian studies on grandi lievitati, published between 2004 to 2012, tracking microbial growth kinetics. One of the papers, like the figurative apple bopping Newton's head, sparked a light-bulb moment, and the sweet starter was born.
How does it work, exactly?
The abridged, TL;DR version: It “works” by essentially turning the fermentation into a yeasted one. It does so by selectively killing off one-half of the sourdough community, the bacteria. The sweet starter works the same as grandi lievitati, by using a bacterium's own physiology against itself.
Bacteria are much smaller than yeast, with a rigid cell wall. If the concentration of solutes in their immediate environment shifts abruptly and drastically enough, the water inside the cells gushes out in the literal blink of an eye. The rigid cell wall stays in place, but their insides literally implode, contracting and tearing away from the cell wall. This is called plasmolysis. It's a great way of killing bacteria, if the change in osmotic pressure is sudden and severe enough. Yeast mostly survive, maintaining higher cell densities, left to grow, albeit slowly, and leaven the remaining “enriched” phases.
The longer version? Well, some background information is necessary.
The “average” sourdough contains about one (1) species each of yeast and bacteria as the “dominant” organism. This simply means that, by weight, each organism accounts for over 99.9% of all organisms present. Here's the thing about the microbial world: Nothing is sterile. Nothing. Another way of saying it is, everything's contaminated, everything contaminates everything, everywhere. Microbes persist. They're survivors, able to withstand unimaginably harsh conditions in a passive, inactive state, for inestimable lengths of time. (Just how long? No one knows. Tens or hundreds of tens of thousands of years; we've discovered instances of hundreds of millions of years. We simply have no idea what the upper or lower bounds are.)
So, that remaining 0.1% of a sourdough community—currently called “satellite members” by microbial ecologists—represents the previously-mentioned “everything.” That 0.1% is incomprehensibly diverse, representing hundreds, thousands, tens of thousands of different taxa. Our current technology likely under-represents what's there and cannot really tell us what they're doing.
We can assume they're persisting, waiting for conditions where “they”—the minority species—can flourish, and become king of the hill. Microbial communities are cutthroat. If something dies, there's always [someone] ready in the wings to take its place. This will come up later, when we discuss acid- or dextran-production in final-dough conditions.
The other startling fact about microbes is their plasticity. They're extremely versatile, mostly because the majority of their molecular evolution occurs horizontally. They can scavenge, share, or passively acquire genetic information from viruses, other microbes, or even the environment. They possess active physiological mechanisms that can “decide” whether to insert functional sequences into their genome. Our macrobial ideas about taxonomy don't apply here; it's best to think about microbes by their community function rather than individual strain or species identity.
How does this relate to sourdough? Well, the “average” sourdough has two very different organisms—ascomycetous yeast and heterofermentative lactobacilli—from entirely separate domains that dominate the community. It's important here to note that there is no particular strain or species that's “endemic” or “native” to a specific fermentation or even place; you'll find both types of organisms dominating most spontaneous or backslopped anaerobic fermentations based upon plant material rich in carbon (sugar) or amino acid (protein) sources. You could say both types of organisms are “pre-adapted” to the conditions found in such fermentations, as their lifestyle revolves around decaying or carbon-rich angiosperm plant material (fruit, flowers, starchy reproductive storage organs, sap, nectar, honeydew) and the guts of insects and other invertebrates associated with those plants or plant material.
It's often stated that the “average” sourdough contains 100 bacterial cells for every 1 yeast cell. This number is slightly confusing, because it leads bakers to mistakenly believe bacteria dominate. Thing is, yeast cells are approximately 100 times the size of bacteria, which means, if quantified by biomass, bacteria and yeast exist in approximately equal quantities. One cell is simply much bigger than the other. In continually-propagated sourdoughs, then, they tend toward equilibrium.
In food and beverage fermentations, yeast produce ethanol and carbon dioxide. They're good at making people drunk and gassy. Heterofermentative bacteria also produce the exact same metabolites, with the addition of organic acids. We know it's easy to make wheat bread using yeast; it's more challenging when bacteria are present. Their organic acids modify the pH of a dough system, activating enzymes in flour that break down gluten. It's doubly challenging when making sweet, enriched products, as most people, myself included, don't like the taste of sweet products that are also sour. Sugar increases the perception of acidity, making it taste even more acidic.
The idea behind the sweet starter is simple: Abruptly move an exponentially-growing microbial community from conditions of minimal stress (say, a mixture of only flour and water) to an extreme environment of intense osmolarity. You do so by mixing this fast-growing inoculum into new-dough conditions that contract the available solvent space and that also increase the solute-to-solvent ratio.
A naïve assumption would be to simply lower the hydration to minimal water amounts, but this approach doesn't work. Why? Here's a good rule of thumb: If you can form a dough out of it, then it'll support microbial growth. That is, it takes about 30% to 33% water to flour weight, like in pasta, to form a cohesive dough. This hydration also corresponds to the minimal hydration at which three separate phenomena materialise: enzymatic activity starts; microbial growth occurs; and electrical conductivity arises.
The simultaneity at which these seemingly unrelated events take place, like an ON/OFF switch has been flipped, tells us something about the way in which water is distributed in dough and batter systems. Foods and beverages are multi-component, multi-phase systems, with heterogeneous structure and distribution of water between the components and phases.
Lean doughs based upon wheat flour thermodynamically separate into four principal phases, each with varying densities. In order (by decreasing moisture concentration), a “dough liquor;” a pentosan gel; a gluten gel; and a starch phase. Depending upon initial water concentration and mixing procedure, an unstable equlibrium is achieved between the phases. Below 30% to 33% hydration (~23% to 25% total moisture) only the last three phases, which contain the large-molecular-weight biopolymers, are hydrated. The “dough liquor,” discontinuous, µm-sized liquid droplets dispersed within a continuous, liquid protein phase, doesn't emerge until this threshold (30% to 33% hydration) is met.
The dough liquor is a dispersed aqueous phase that represents 5% to 20% the total dough weight. It contains all the sugars, salts, minerals, amino acids, water-soluble proteins (albumins, globulins), soluble polysaccharides (pentosans, damaged starch), ethanol, organic acids, dissolved gases, microbial cells, and lipids. The dry matter content of the aqueous phase remains stable, at 5% to 15%, between dough hydrations of 30-33% up to a total hydration of ~122-143%. The latter means that, up to hydrations of about 122-143% (which represents the dough-to-batter transition), the size of the dispersed aqueous phase increases quasi-linearly.
(For a deeper discussion on dough structure, phase separation, and its impacts on rheology and fermentation, please see my grAiNZ talk on YouTube, “Like With Like: How & Where Things in Doughs & Batter Go,” embedded below.)
The dispersed aqueous phase represents the total solvent volume of a dough system. As hydration increases up to the “batter” limit (at which phase inversion occurs, and the dispersed aqueous phase becomes continuous), the size of the dispersed aqueous phase droplets increases. By decreasing total solvent, we also decrease droplet size, which, for geometric reasons, allows the amount of osmotic pressure exerted to scale exponentially (due to greater surface-to-volume ratios). Added sugars, salts, soluble proteins and polysaccharides, and fats end up within the dispersed aqueous phase.
In this way—by decreasing total solvent volume and by increasing solute concentration within that phase—osmotic pressure is greatly increased.
Of course, water is not just a solvent; it's also a plasticiser. Plasticisers are small molecules that attractively interact with stiff, large-molecular-weight biopolymers, increasing their flexibility and total volume. All biological material is structured by one of two biopolymer types, proteins and polysaccharides. Without plasticising agents, like water, they'd remain rigid and incapable of movement. The same goes for doughs.
Water is nature's most efficient plasticising agent. When you decrease solvent volume (and hence water content), you still need to keep plasticiser volume constant, or even increase it. This allows flexibility of a dough's structuring material, and we can do so by adding sugars, soluble proteins (from egg), and fats (from egg, dairy, or plant sources). Fats, though insoluble, are dispersed within the aqueous-phase droplets, usually seeking out liquid-liquid or liquid-gas interfaces. Depending upon whether the fat is solid or liquid, it can help stabilise dissolved gas bubbles. Egg proteins, both from the white and yolk, participate in biopolymer plasticisation and in continuous network formation before and after thermosetting.
You mention that a “significant amount of sugar is still required in the final dough as well.” Is this simply because—as you mention later on—high sugar concentrations help to avoid acidity and minimize “cheesy off-flavors” (something I despise in enriched sourdoughs myself), or is there something more to it? I’m not sure many of those who've taken your method and run with it are aware of this.
Just because you severely diminish the dominant bacterial population during one step of processing, it doesn't mean bacteria aren't there in another. There still may be survivors capable of growth in the successive fermentative stages, persistent cells leftover from the dominant population, or bacterial species that arise from the satellite community existing at the fringes.
It's best to imagine bacterial growth as a constant onslaught, like a bad zombie film. They're unrelenting. Remember, everything's contaminated. Just because you kill a significant amount in one step doesn't mean you've eradicated them all. It's a never-ending battle. You need quite a bit of sugar in final-dough conditions—at least 20% to final-dough flour, but more's better, much more—as well as ingredients that, either, reduce solvent volume and/or increase plasticiser volume. Think salts, fats, proteins and sugars. The “average” panettone contains 35% sucrose and 5% honey to total flour weight, much sweeter than amateur bakers realise.
You mention several times that the amount of prefermented flour in the final dough has an effect on the result, but other than seeming to favor lower amounts, you don’t really articulate why the PFF% matters, and what happens when one exceeds the ideal amount. Can you say more about PFF% and sweet starters? This is also something nobody else who promotes this approach mentions, as far as I know.
Unicellular microbes grow as a community. They continually take stock of their immediate surroundings (including resource concentrations, who their neighbours are and what they're doing), predict future conditions, and “talk” to each other. This allows cells of “similar” type to regulate and coordinate growth, ensuring survival of at least part of the group. Microbes are constantly tinkering with their own morphology, structure, phenotype, protein expression, and so on, tuning it through a variety of molecular mechanisms to best “match” their past, present, and future conditions. The cells in your bodily tissues do this, too, but you're not aware of it.
PFF% or rate of inoculation (flour-only basis) is one way of expressing initial cell density at the start of a “new” growth phase (or an environmental flux), when fresh solvent space and resources have been provided. Initial cell density is one of the factors that determines what microbes of a similar type will “do” when presented with uncertain or new conditions. It influences the community-wide response: possible divisions of labour, chosen phenotype(s), metabolic or growth strategy, genetic regulation and expression, etc. It thus impacts lag phase, growth characteristics, metabolites, etc.
At high dilution rates (low PFF% or inoculation; high multiplicative factor), lag phase sharply increases. Conversely, at low enough dilution rates (high PFF% or inoculation; low multiplicative factor), cells may “choose” phenotypes associated with stationary phases of growth, when metabolism occurs but not necessarily growth (biomass increase). Such phenotypes direct more energy derived from metabolism toward stress responses, as nutrient concentrations are becoming depleted and environmental conditions harsher. Cells in stationary growth phases have much higher survival rates against external stresses or perturbations, including shifts in osmolality.
It's important to bear in mind that “growth phases” (lag, exponential, stationary, death) are an idealised model created by humans, for humans. In reality, microbial phenotype is a spatiotemporal response that exists as an ever-changing continuum. That is, it's not just a time-based reaction, as is often depicted in baking texts. Cells may “enter” stationary phases (phenotype is probably the better word here versus “phase” or “stage;” “stationary” meaning metabolically-active but with little biomass increase and increased expression of molecular stress mechanisms) as a response to conditions in time or space. The latter means acid may be produced, regardless of whether there's growth (biomass increase), because bacterial metabolism is still switched “on” and generating energy. The oxidised, metabolic byproducts (like acids) still need to be exported somewhere.
A sweet spot exists in relation to PFF% and final-dough conditions, one that can only be determined empirically, i.e. via trial-and-error. There are several considerations that help decide how much PFF% to use: processing temperatures; maintenance-starter elaboration; degree of enrichment; etc.
Generally speaking, products made from a sweeter starter work best by prefermenting ~15% ± 2.5% of the flour, with lower degrees of in-dough enrichment relative to grandi lievitati, i.e., ≤ 30% added sugar, fat, and egg or egg yolk to total flour weight.
Lower processing temperatures—24° to 26°C (75˚ to 78˚F)—are best for maintenance and sweet-starter elaboration, and 26° to 28°C (78˚ to 82˚F) for final-dough conditions. (The increase in temperature matches the increase in degree of enrichment.)
For a sweet-starter build, I recommend using a liquid starter, which, for amateur or small, independent bakers, is easier to mix. It's best to use at least two or three, successive, back-to-back refreshments, similar to grandi lievitati. A feeding ratio of 1:1:1 works fine, for 3 to 6 hours of total fermentation time. Use only low-extraction, roller-milled flour, and fermentation temperatures of 24° to 26°C (75˚ to 78˚F).
The actual starter build is where I see the majority of amateur bakers mess up. Online, at this point, it's the blind leading the blind. There was one very big social-media influencer who's popularised the sweet starter. Like most amateurs, she's changed the recommended ratios, lowering the sugar amounts. Ironically, this person has flown around the world to meet me, and take classes from me.
So, now, I see other influencers copy-pasting her recommended sweet starter ratios, who further influence more influencers. All are using insufficient sugar amounts, which will have the opposite intended effect. At the levels I see recommended, it will actively increase acid, particularly acetic acid (because sucrose is hydrolysed to glucose and fructose, and the latter is the surest way of increasing acetic acid).
I want to say this loudly and clearly: Feel free to use whichever ratios you want. However, if you want a reliably non-sour, light product, please stick to the recommended guidelines. Nearly every bakery on the planet (on four continents) using a sweet starter in production has a direct connection to me. They've either worked or staged with me, or I created all the formulas and processes at these bakeries.
I created, pioneered, and thoroughly tested every imaginable permutation, a percent at a time, using different ratios and temperatures for elaborating a sweet starter, changing only one variable at a time to arrive at the “correct” inhibitory concentrations. Funnily enough, the results very much mirror those used in the first yellow dough of most grandi lievitati.
As already mentioned, the “correct” inhibitory concentrations can only be determined empirically. It's hard for me to explain to amateur bakers that using lower amounts of sugar has the opposite effect. (There's a particular lack of humility here. I'm currently consulting at a bakery that uses a sweet starter for all pastry. We are making more croissants in one day than any amateur will make in a lifetime. Not all things work. I've already done the hard yards for you. If you want a less sweet product, use yeast or a fermented fruit water.)
For a sweet-starter build, it's best to use a multiplicative factor of 4 to 5. This is the ratio of flour in the maintenance starter to sweet-starter flour. As outliers, you can use 3 or 6, but other alterations in processing or ratios need to be made.
The “best” sugar amount is 25% ± 5% to total flour weight. For hydration, I recommend 42% to 45%. These latter values are calculated against total water and flour in the starter build.
A “theoretical” starter build would look something like:
100% T-55 or all-purpose1 flour
Enough water to equal 42% to 45% hydration
30% sugar2
20% continuation starter, expressed on a flour-only basis (+ its water)
So, if using a continuation starter hydrated at 100%, it'd look something like:
100% T-55 or all-purpose flour
40% continuation starter3
30.4% - 34% water
30% sugar
Ferment at 24–26°C (75–78˚F) for 10 to 11 hours.
You mention the pre-bake stability of doughs and products thanks to the sweet starter. Can you explain what causes this effect? Is it simply the lack of acidity/bacterial activity, or something else?
When formulated and processed correctly, unfermented products made with a sweet starter have a much longer “use-by” date relative to grandi lievitati. There are two principal reasons. The first is obvious: Less prefermented flour. But this still doesn't explain why you can refrigerate such products for up to three or four days in shape, with little compromise to volume or texture, and still have no sourness. The presence of abundant reducing sugars and the continual cycling between hot and cold environments causes yeast to accumulate compatible solutes, small molecules that increase cold-stress survival.
I also suspect the molecular nature of the in-dough plasticisers play a huge role: you're less likely to have long-term storage success if you are using doughs rich in “pure” fats (butter, oils, margarine), approximately 30% to total flour weight. Once refrigerated, liquid fats solidify, becoming crystalline. Fat solidification would have serious consequences for increasing pressure within the dough's dispersed aqueous phase, micrometre-sized droplets where microbes “live.” Fats exist within such dispersed, liquid droplets, or at liquid interfaces. (Egg-yolk fats are much more complex in structure and composition and present in much lower amounts.)
I assume there’s no reason bulk doughs wouldn’t also be equally stable, no? I tend to chill all of my enriched doughs until firm before shaping, so if you could hold them in the fridge like that for up to 3 days, that would be another reason to opt for a sweet starter.
I wouldn't personally store doughs refrigerated in bulk for this long, simply because of temperature gradients that arise due to lower surface-to-volume ratios. By storing products in shape, you allow for greater surface area, creating a more stable product during cold storage. This particularly becomes a more important consideration in commercial environments where refrigeration temperatures continually fluctuate.4
Like grandi lievitati products, sweet starter enriched bread are far more shelf-stable than yeasted ones, all else being equal. In the case of grandi lievitati, it’s the production of dextrans during the multi-dough fermentation that create this effect. Are dextrans to explain for the longevity of breads produced with a single-stage sweet starter? Or is it something else?
Dextrans are a peculiar obsession of Thomas Teffri-Chambelland's, the owner and founder of EIDB. Baking schools are interesting. They're not truly meritocractic, because anybody who can afford the (exorbitant) fees passes. This means the curriculum has to be as cookie-cutter and as simplified as possible, with no student left behind. That's fair enough, as his school is a business, after all. Bottom line still matters. Thomas does a very good job of introducing complete beginners to alternative ingredients and methods of processing. The purity of his approach should be commended. It's something I greatly admire.
However, I'm not entirely convinced by the way he incorporates scientific material into his program. First, he doesn't get a lot of the science “right,” particularly on subjects dealing with anything that's alive. This is particularly evident in his first book, which seemed as if he didn't review a lot of the related scientific research on his subjects he discussed, and slightly less so in his second book. Dextrans are a great example, one I'll comment on below. My second issue is his insistence on using tools like pH metres to quantify fermentation rather than using sensorial cues, as bakers have done since the beginning of time.
Living systems are infinitely complex, often with more exceptions than “rules.” Prokaryotic microbes even more so. There's a reductive literalism to what he teaches. (To be fair, he's not the only one guilty of this.)
Dextrans are an illustrative example. Dextrans are a class of extracellular microbial polysaccharides (“exopolysaccharides,” or “EPS”) produced by certain strains and/or species of lactic acid bacteria (cocci from Streptococcus, Leuconostoc, or Weissella genera).
EPS are biopolymers that function as a protective matrix for cells growing as a colony. The secreted EPS surrounds the cells, acting as a biofilm in which they're embedded. Biofilm formation is not unique to the particular aforementioned lactobacilli, or even to lactobilli in general. EPS production is a widespread trait in the microbial world.
Functionally, all EPS are similar in fermented food and beverage systems. Dextrans are but one kind of EPS. I'm not sure why Thomas focuses only on this one particular class, as there are a multitude of bacterial polysaccharides produced in sourdoughs. What's more, there's no guarantee dextran-producers will be present in any individual sourdough system. They were in his the one time he looked. There are plenty of other types of EPS that may or may not be produced; if they are, they'll be fuctionally equivalent.
Product shelf life is influenced by post-bake moisture content and water-transfer kinetics; concentration of non-water plasticisers that are functional at storage temperature; molecular species that retard starch retrogradation; product density; etc.
Most home bakers do not use a 2- or 3-stage build prior to the sweet starter build (I’d bet most people do a single build). Is this simply to ensure the starter is vigorous before it goes into the sweet starter?
Successive, short feeds using high temperatures and high rates of inoculation (multiplicative factor of ≈1.5), like those for grandi lievitati, have a three-fold effect. First, they ensure vigour, increasing specific growth rate. Second, they decrease total acid in the system, since acid accumulation is correlated with time. Third and most importantly, it shifts cell morphology. Unicellular microbes actively “tune” their physiology to match external conditions. They do this on a continual basis. Amongst the most salient to microbial success is membrane structure, as this is what separates living (a cell and its interior) from non-living (everything outside of it).
Using a back-to-back refreshment schema, as outlined above, causes a “thinning” of the bacterial membrane, a trade-off from increased growth rate and cell volume. Their weakened membrane leaves them more susceptible to death or injury due to playsmolysis. Yeast, on the other hand, have the opposite experience. The short refreshments leaves a higher percentage of mother cells with thickened membrane structures in the yeast population, improving their survival during sudden, drastic shifts in osmolarity.
Recently I have noticed some folks on Instagram using a “liquid” sweet starter, of ~100% hydration. You suggest that would have the opposite effect (increased bacterial activity, increased acid), since the osmolarity would not be conducive to bacterial cell death, and the presence of sucrose would increase the amount of acetic acid produced. Are you saying that a liquid sweet starter would actually be worse than an unsweetened one of any hydration?
It's not that there's increased bacterial activity under more liquid conditions, sweetened or not; rather, there's a decreased rate of death or injury due to plasmolysis. (You basically say the same thing, but I want to make clear the difference. Bacterial growth rate and total cell density would, at most, match a control, unsweetened baseline, never surpassing it. Adding sugar beyond a certain amount will only decrease both. The question is, how to maximise the impact of the added sugar, to prevent and totally inhibit bacterial growth?)
A liquid sweet starter is not bad, per se. It's better than an unsweetened one of any hydration, assuming sugar levels are high enough for growth inhibition and/or to plasmolyse bacterial cells. It's important to use successive, short, high-inoculation refreshments as the starter becomes more liquid.
One variation on the liquid sweet starter, created and used quite successfully in a real-world bakery production, is a young sweet starter. This permutation still uses 25% sugar to total flour weight. It was first discovered and refined by Maryaasha Werdiger of Zelda Bakery in Melbourne, Australia, and used to leaven her awesome babka. This same approach is also used by the extremely talented Em Hart at Damsel Bakery in Crieff, Scotland. Em makes a wonderful bun dough, lightly enriched with butter and egg, that contains more than 50% high-extraction flour. The final product has no perceptible sourness.
This approach, using a young sweet starter, is best for doughs that contain low degrees of enrichment, particularly solid fat. I'm guessing you want to use less than 20% solid fat to total flour weight, as well as pushing egg level up, to allow greater extensibility, elasticity, and air-bubble stabilisation. This approach also allows you to decrease sugar levels in the final dough—Maryaasha and Em both use less than 10% to final-dough flour weight.
One note: A young liquid sweet starter can likely not be used for days-refrigerated storage of the shaped loaves.
I’ve also seen people maintaining a sweet starter, rather than building one off of an unsweetened one. That’s not really a viable approach, right? Seems like the yeast would suffer if you held the sweet starter for any length of time after it has matured.
It is a viable approach if you want to increase stress tolerance of your culture. It will have the exact opposite effect seen in sweet starters or in the first yellow-dough stages of grandi lievitati. You'll have more cells survive, intact and viable, ready to acidify your remaining stages. If that's your goal, then, by all means do so. Remember: Unicellular microbes continually “tune” their physiology to best deal with the biotic and abiotic stresses of their surrounding environment. If you use a stepwise or granular introduction of stress—say, maintaining the culture continually with sugar—you will select for cells, both bacteria and yeast, adapted for growth and reproduction in those conditions. Also note I only mentioned stress generally. Stress proteins and responses in microbes tend to be multi-functional and non-specific, allowing increased resistance to a variety of stresses, not just one.
What is the explanation for the long final fermentation times of sweet starter doughs? Is it simply that yeast cell density is also compromised by the presence of the sugar?
I think this question shows a bit of a category mistake in your thinking. As above, it's probably not appropriate to think in terms of time (kinetics) alone. Space (thermodynamics) matters, too. Length of fermentation is a consequence of all processing parameters. Cell density plays a huge part in all this. The sweet starter and products elaborated from it work best using lower PFF%. As such, the entire process takes longer, although, kinetically, they would be equivalent to rates found in grandi lievitati.
What you gain in flexibility is accounted for somewhere else in the process, say, as a loss in immediacy of processing.
If it is dextrans that give SS breads longevity and it is low yeast cell count to blame for long fermentation times, could you get the same effect with a faster final proof by dosing the final dough with commercial yeast? Or is the long final fermentation somehow essential to the result?
Feel free to use commercial yeast. (My favourite croissants are yeasted, no preferments. There's as much an art to making wonderful, well-fermented yeasted products as there are ones made entirely from sourdough.) The resulting product will be different in keeping quality and depth of flavor.
Follow-up questions [added 5/16/25]
After thinking about and working with the SSS method awhile, I had some further questions for Ian, which he answered for me over email. Some of this is very technical, but there's loads of useful intel here.
Why do you recommend lower-protein flours for the starters and final dough? Does the protein content influence acid production too, or is that just a matter of preference?
I recommend refined flours with moderate protein content for both the continuation starter and final dough, not because of protein’s role in buffering acid, but because of how it influences rheology, extensibility, solvent phase quality, and microbial accessibility.
Stronger flours tend to contain higher concentrations of insoluble high molecular weight glutenins. Under mixing, these glutenins can form dense, elastic aggregates that resist deformation. This creates:
- tighter, more bucky doughs
- less extensibility
- increased tenacity
- reduced motion under fermentation pressure
Strong flours also generally contain more damaged starch and finer particles, both of which increase dough viscosity. Damaged starch absorbs water more aggressively, which reduces the accessible aqueous solvent phase available for both gluten plasticization and microbial diffusion.
In sweet starter systems—particularly in final doughs with low prefermented flour and moderate enrichment—water is the primary plasticizer and microbial solvent. Its quality (mobility, distribution, availability) matters more than its quantity. Stronger flours tend to lock that water into tighter, less-mobile domains. The result: doughs that resist fermentation-driven expansion, produce less gas retention, and are slower or less responsive in proof.
Acid production itself is not significantly influenced by protein content in refined flours. Buffering capacity may be marginally higher in stronger or wholegrain flours, but that’s not the core concern in the stiff sweet starter context. The main issue is that high-protein flours tend to produce rheologies that are too tight, too elastic, and not conducive to the goals of the SSS framework.
The ideal flour:
- has enough protein to form a coherent network
- but not so much that it produces excessive tenacity, internal resistance, or microphase compartmentalisation
This balance supports:
- microbial access
- extensibility under moderate enrichment
- gas trapping
- structured softness and post-bake expansion
So the recommendation isn’t a matter of preference—it’s grounded in how formulation, structure, and phase behavior determine the viability of both the starter and the final dough in the SSS process.
One way to express this balance is through ρ′, a structural dominance index defined as:
ρ′=insoluble structureinsoluble structure+plasticisers\rho' = \frac{\text{insoluble structure}}{\text{insoluble structure} + \text{plasticisers}}ρ′=insoluble structure+plasticisersinsoluble structure
Where plasticisers include all water (from water, egg, butter), sugars, fats, and soluble proteins. ρ′ captures the fraction of the dough system dominated by the structural gel network, relative to total phase-softening elements.
higher ρ′ = tighter, more elastic, more resistant to deformation
lower ρ′ = softer, more extensible, more plasticised
using this ratio, we see:
- lean dough made with 11.5% protein flour = 8.56%
- croissant made with sweet starter & 13.5% protein flour = 7.85%
- brioche made with sweet starter & 11.5% protein flour = 5.76%
- “average” panettone = 5.05%
This confirms: lean > brioche made with sweet starter > “average” panettone, by structural dominance. Flours that are too strong shift this ratio upward, creating excessive resistance and reducing responsiveness—exactly the opposite of what the sweet starter system needs.
What are the tell-tale signs of a long-enough bulk fermentation? or does it not really matter, provided the total ambient fermentation time (bulk + shaped) is sufficient? To put it another way: is there a minimum required bulk fermentation time before the dough is safe to retard?
In sweet starter systems, bulk fermentation is not evaluated by visible volume gain. Most doughs made with the sweet starter—especially those with moderate to high enrichment—barely expand during bulk, even after 8–10 hours at ambient temperature. This is not a defect, it's a feature of how these doughs are structured.
Movement is a function of rheology, not time. and rheology is governed by:
- formulation (sugar, salt, egg, fat, water)
- mixing regime (sequence, speed, temperature, hydration staging)
- resistance to extension and tenacity, shaped by protein strength, starch damage, flour particle size, and fat phase behavior
- amount and distribution of accessible aqueous solvent, which defines internal phase fluidity and microbial mobility
- prefermented flour, which controls the initial fermentative load
High enrichment, plastic fats, and lower water levels increase osmotic stress and lock up the dough’s internal solvent into tighter, phase-separated microdomains. in these systems, even an active starter doesn’t create volume early, because the dough resists expansion. the microbes are working, but the structure doesn’t yield.
So: bulk fermentation is for internal conditioning, not leavening. The goal is not rise, but readiness. A bulk-fermented sweet starter dough, even one that hasn’t moved, will feel:
- softer at the surface
- more extensible
- slightly smoother
- more cohesive in the hand
This softening reflects the starter’s early activity:
- diffusion of acids and volatiles
- pressure equilibration
- restructuring of semi-gelled or stressed interfaces
- solvent re-partitioning
Bulk fermentation must last long enough for this to begin. Otherwise, the dough will be sluggish or unresponsive during final proof. For most SSS doughs—especially those with low prefermented flour and moderate enrichment—6–12 hours at ~24–26°C (75–78˚F) is the minimum. Even then, expansion might not be visible.
Retarding too early is risky. Though the cold slows acid development and preserves structure, it also stalls internal rebalancing if it hasn’t already started. This is why the minimum bulk time matters—not to inflate the dough, but to initiate its capacity to respond later.
Only some doughs made with the SSS move during bulk. A brioche made with the sweet starter, for example, typically composed of ~35% water, ~33% egg, ~25% sugar, and <15% plastic fat, may show some rise—especially when mixed with a paddle and folded regularly. This dough’s internal structure is softer and more open. its ρ′ is around 5.76%, lower than lean dough (8.56%) and higher than panettone (4.85–5.05%), indicating a relatively balanced plasticizer-to-structure ratio. These are edge-case doughs where both movement and readiness may coincide.
But for everything else: movement ≠ fermentation quality. You don’t judge readiness by rise. you judge it by final dough performance. Bulk is a setup phase. the dough may not move—but it must still rest.
Is the total fermentation time of the dough tied to the percentage of sugar and degree of enrichment (along with temperature and humidity)? Meaning—provided you have a robust starter and maintain a constant temperature and humidity—it will vary only based on formulation? i’ve had a few doughs move pretty quickly, particularly in the final fermentation stages, and have found it safer to retard overnight and commence the final fermentation in the morning, to avoid risk of overproofing, so i want to understand why that would be the case.
Yes, total fermentation time is deeply tied to formulation—but not just to enrichment levels in isolation. It depends on how that formulation structures the dough’s internal environment. Enrichment (sugar, fat, salt, egg) reshapes the physical and thermodynamic conditions under which fermentation occurs.
Microbial kinetics are not only modulated by temperature and humidity; they are mediated by:
- the aqueous solvent’s availability, quality, and phase distribution
- the microbial cell density (PFF) and its spatial dispersal
- the dough’s resistance to deformation (elasticity and viscosity)
- the plasticizer–structure ratio, expressed here as ρ′
In the sweet starter system, where prefermented flour is low and structure is sensitive to solvent partitioning, these factors become dominant. A dough’s movement doesn’t reflect microbial activity alone—it reflects whether the dough is structurally capable of responding.
For example:
- a lean dough (ρ′ = 8.56%) with high water and no osmotic stress moves early and consistently
- a brioche made with sweet starter (ρ′ = 5.76%) may show moderate movement
- a panettone (ρ′ = 4.85–5.05%) or croissant (ρ′ = 7.85%) often moves little or not at all during bulk
- in croissant, plasticizers and cold butter increase extensibility but delay visible rise until the final stages
Movement often accelerates in the later proof stage because:
- the dough softens as acid accumulates and gel interfaces relax
- the microbes encounter less phase resistance
- solvent compartments re-partition, reducing osmotic pressure
- the system crosses a threshold from “fermenting without response” to “responsive to internal pressure”
Retarding overnight and continuing the final fermentation in the morning works because it gives the system time to stabilize before further acidification, while preserving gas potential. This strategy is particularly useful for high-stress doughs, where microbial activity occurs early, but expansion occurs late.
Formulation sets this whole trajectory. Once the starter is healthy and temperature is stable, the variation in fermentation timing is no longer biological—it’s physical. it’s about the readiness of the dough’s internal structure to allow expansion. High-stress, plasticized, or emulsifier-rich doughs often take longer to reach this responsive state. and once they do, they may move very quickly.
So yes—fermentation time varies by formulation, but not because time itself is slower or faster. It varies because each dough has its own timeline for structural accessibility. ρ′ helps track this indirectly: higher values = earlier response; lower values = slower softening, delayed expansion, longer proof.
You say the ideal range for prefermented flour in the final dough is 15±2.5%. how would you decide where to land within this range? Is it based on the formulation of the dough, or simply a question of desired fermentation rate?
The ideal prefermented flour (PFF) range—typically 12.5–17.5%—is not about fermentation speed alone. it’s about the stress landscape the microbes will encounter once in the dough, and how much biological effort is required to overcome it.
The primary determinant is not enrichment per se, but how that enrichment affects aqueous solvent availability and phase accessibility. aqueous solvent is the medium for microbial viability, diffusion, and metabolic function. When it becomes limited, structurally compartmentalized, or osmotically hostile, microbial performance drops—unless compensated for by increasing the PFF.
Formally: more stress = more PFF
Stress arises from:
- sugar (osmotic)
- plastic fats (phase separation, immobilisation)
- salt (ionic, ionic–osmotic coupling)
- emulsifiers and proteins (droplet densification and micro-compartmentalisation)
- low water and/or restructuring from mixing
- also: the viscosity and extensibility of the dough, determined by starch damage, flour particle size, and protein behavior
The total stress is not just additive—it’s structural. The more phase-separated, bound, or discontinuous the aqueous environment becomes, the more difficult it is for a limited microbial population to proliferate and equilibrate.
This is why PFF is best understood not as a driver of rate, but as a threshold-setting variable. a higher pff gives:
- higher initial cell density
- better coverage across dispersed aqueous compartments
- faster rebalancing of pH and metabolite diffusion
- reduced lag time for movement during proof
- greater control over acid levels, especially with longer proof times
Conversely, a lower PFF:
- increases susceptibility to structural inhibition
- slows acid accumulation
- results in a tighter, more elastic dough for longer
- risks underdevelopment if bulk is too short
Also, spatial distribution matters. When PFF is low, microbial density is low. in highly emulsified or phase-separated doughs, this can result in spatial discontinuity—regions of dough that lag or behave differently depending on whether they contain starter-accessible solvent. This becomes more critical the more dispersed the aqueous phase becomes.
Mixing time and dough rest must also be considered. Time-dependent restructuring of the dough liquor phase (e.g. through folding or rest) can soften structural discontinuities and reduce the necessary pff. But this only works if the dough is extensible enough to allow restructuring.
Examples:
- lean dough: low stress, high solvent, high ρ′ → PFF near 12.5% suffices
- brioche with sweet starter: moderate stress, moderate solvent, ρ′ ~5.76% → PFF around 15–16%
- panettone: high stress, low solvent, ρ′ ~4.85–5.05% → PFF closer to 17.5% is safer
- croissant: high structural resistance from lamination (ρ′ = 7.85%), but relatively low enrichment in the dough base → PFF around 14–15% is typical
PFF should be scaled relative to:
- the quantity and accessibility of aqueous solvent
- the phase architecture and droplet stress
- the type and intensity of enrichment stressors
- and how well the starter was built to tolerate these stressors
This is why arbitrary rules about prefermented flour don’t hold up across doughs. PFF is not a tuning knob—it’s a structural buffer. Its role is to ensure that the starter’s activity is not undermined by the physical architecture it’s dropped into. The more hostile the landscape, the more backup it needs.
T55 is the French designation for a refined flour comparable to American high-protein all-purpose and bread flours. ↩
Sugar as in sucrose, aka granulated white sugar or its equivalent. ↩
I.e., the last build from the 100%-hydration liquid levain builds done prior to building the sweet starter. ↩
I think this would not apply when working with home-baker scales of doughs only 1x to 5x the final shaped weight. ↩
wordloaf Newsletter
Join the newsletter to receive the latest updates in your inbox.