Flowers and flours
An interview with sourdough starter expert Debra Wink
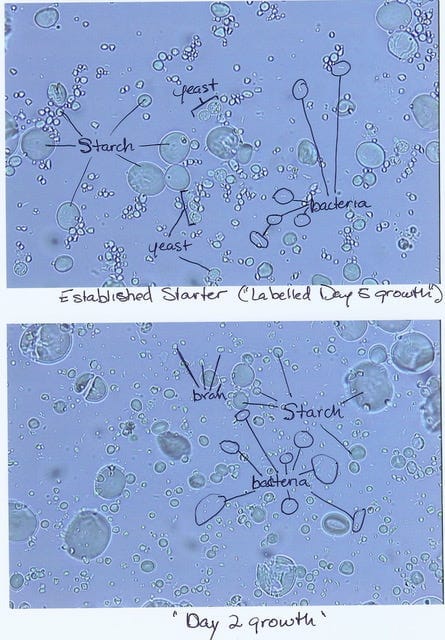
Table of Contents
Hey all, it’s Andrew here, taking over the take-over from Amy briefly to share an interview that I did with the Internet’s number one sourdough-starter expert, Debra Wink. It’s a long one (as most of my Wordloaf interviews seem to be), but well worth your time, I promise. It was well worth my time to edit too, even though I’m supposed to be hard at work on my book right now, because Debra has influenced my thinking on sourdough starter creation and maintenance in profound and fundamental ways.
Debra has spent more than 20 years teaching home and professional bakers the science of sourdough, and more importantly, helping budding sourdough bakers diagnose and cure their ailing and failing starters. During that time, she’s been responsible for several key innovations in the sourdough-starter creation process, including the use of pineapple juice in place of water in the initial starter mixture, to produce a pH that is favorable to the growth of lactic acid bacteria and sourdough yeasts and inhospitable to the microorganisms that often cause fledgling starters to go through a stinky, unpleasant adolescent phase.
I loved this conversation, and I think you will too.
—Andrew
Andrew: Tell me about your career as a microbiologist, and how it fits into your exploration of bread baking. My sense is those two things are separate but they came together in sourdough.
Debra: Full disclosure, I do have a degree in microbiology, but I went on to study clinical laboratory science and become a registered medical technologist. I spent 20 years working in clinical laboratories testing blood and other kinds of human samples. Not very much of my work involved bacteriology and mycology, although I’m trained in those areas too. I worked mainly in hematology, another branch of microbiology.
My start in bread baking came about through the rise of the Internet. Actually, without it, I don't think it ever would have happened. Back in the early 2000s King Arthur started their Baking Circle, an online forum, and I joined soon after it began. This was also during the rise of The Food Network. In its early days, there were a lot of great basic shows on all sorts of subjects. One of the baking shows had Daniel Leader on as a guest, and he was demonstrating sourdough and how to make a sourdough starter. I come from a family that bakes, I grew up baking and I have this love of microbiology. So those two things came together in sourdough. I was fascinated by the whole process of starting a starter from just flour and water. Before Daniel Leader I didn't know you could do that. So I tried my first sourdough starter. It went a little bit bumpy, but that didn't really faze me too much because I was comfortable working with cultures. I started a second one with a little bit more intuition, I suppose, and got it up and running.
Around the same time there were many people on the King Arthur baking forum posting that they were having all kinds of problems with Peter Reinhart's sourdough starter process. At that time, his book The Bread Baker’s Apprentice was very popular, and people who were starting in sourdough were using that as their introduction and having so much trouble with it.
So it just came about organically that I started answering questions about microbes and how they live, grow, and multiply. And I started helping people with their starters, especially the one person who started the thread that launched it all. She was following the BBA procedure over and over and just kept getting the same frustrating result. I told her I would do the same to see it for myself. I got exactly the same result that she did and that was exciting to me—wherever there's a pattern that's repeatable, there's something going on that’s worth figuring out.
So that's how I got into looking under the hood, so to speak. I hadn't taken any of these things to work and looked at them under the microscope before that. I was a little bit perplexed as to why the yeast were dying off at the same place every time for her, for me, and for several other people who chimed in. It was happening all over—so many people were getting the same result, but few were getting the result described in the book.
I began looking at starters under the microscope every day through the whole process. And that was where I first found that there weren't actually any active yeast growing in it in the early stages. It was all bacteria. I would scan each sample for up to 30 minutes—I called it “yeast-checking”—just one field after another and never see any active yeast. That raised the question, if there's yeast in the flour, why aren't they growing?
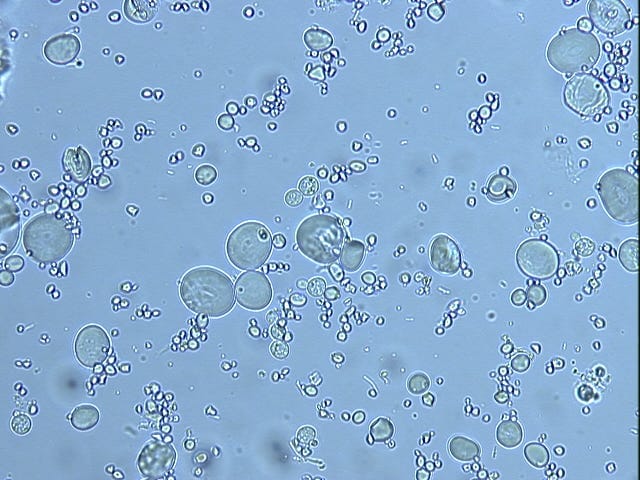
The thing about yeast is that when they are in their dormant form, they're mostly dehydrated. They have very little water in them, so under the microscope they refract light just like a starch crystal. And they're about the same size as the smaller starch crystals too. I didn't have the special stains that you would use to be able to tell them from starch. But I knew they were there, it's just that they weren't in their easier-to-spot active form. Once they become active, they are easy to find everywhere and they fill out the culture very quickly. So it was really easy to find yeast once a starter began rising. It was a really interesting process to see.
But it was tracking the pH of the starters that finally helped me figure out what was going on. That and going back to microbiology textbooks to get a clue for what causes spores to germinate. Unfortunately, there were never any really definitive answers to that for sourdough yeasts. But time after time I noticed that the yeast would start growing only after the pH fell to a certain point, and that seemed to be an important key to success.
The beauty of it was once we found the right acidity, we could bypass all the undesirable stuff in the beginning that was slowing down the process. It was really nice for people to be able to get their starters up and running easily, and without all the stink and angst in the beginning.
It sounds like the problems people were having with the Reinhart method or any other method was not that they weren't on the right track, it's that they were looking for something that wasn't going to be there yet. Assuming it should be there made them think they were failing.
Yes. Bakers like to follow instructions and do and measure things precisely. Unfortunately, none of that matters where Mother Nature is involved. Also, most of the procedures that were being written at the time were by people like Peter Reinhart or other professional bakers who were used to baking with sourdough on a daily basis, so their kitchens were probably full of these microorganisms that were just ready to go. Things progress in their kitchens or bakeries pretty quickly because they don't have to wait for the pH to fall and for things to wake up. It's pretty much already being inoculated into their flour-water mixtures from the start.
People trying to follow this procedure at home who have never made sourdough before—or not much sourdough—don't have all those microbes in and around their kitchens to spur their starters along. Theirs follow a different path, and none of the sourdough procedures that were written at the time allowed for that, or told people what to do when things didn't go as described. In fact, some procedures even said, If it doesn't do this by such and such a time, it's failed, start over. If you keep just starting over, you're never going to get past that hump. So it became about trying to lead people through to get over the hump and have some faith that it was going to happen. It just wasn't going to happen in quite the same way as the books were promising.
So you recognized that pH was a key factor, and that it had to fall below a certain level in order for the yeast to activate and some of those early microbe arrivals to die off. What gave you the idea to use an acid from the beginning to set the pH?
It was looking at the patterns and seeing that certain things happened at certain pHs. For instance, the gassy bacteria stop growing when the pH drops below 4.5. Once I found out that it was a Leuconostoc—mine was Lc.citrium—I looked them up in a reference book to find their pH range. Almost none of the leuconostocs will grow at a pH less than 4.8, so it fit perfectly with what I was seeing. When the pH only dropped to 5.0, gassy bacteria grew; but when the pH dropped to 4.5, they were quiet. The acids that we trialed were just to get past that point, so that Leuconostoc and company wouldn't grow and delay things.
But the yeast weren't activating for us until the pH dropped even lower than that. I never saw active yeast in any of mine until the pH dropped below 4.0. That's not to say that all yeast have the exact same requirement, that's just where I was on the pH scale. And for all the people that I coached along the way, their starters followed the same pattern. So I was having people taste their starters, because even though they didn't have pH paper, they had a tongue that could tell whether the starter was getting nice and sour or not. It's quite tart once it gets below 4.0.
So pineapple juice was the acid of choice not because it was the only one that worked, but because it was a perfect choice and convenient in other ways?
Yeah, it just happened to be the most popular. The woman who was first to try it loved the results. So she started spreading the word and it just took off. But I got excellent results with vitamin C (ascorbic acid) too. That one was a little bit more challenging to work with when using vitamin supplement pills, because some of the ones on the drugstore shelf are buffered, which made it hard to get the pH in the target range. But if you have pure ascorbic acid powder on hand, it works really great too, at a rate of ½ teaspoon of ascorbic acid powder per cup of flour (1.8 - 2% in baker's percentage).
Other fruit juices can work too. Orange juice and apple cider (not vinegar) were popular because some people didn't like pineapple juice and didn't want to buy it. You just have to use them for more than one day because the pH isn't quite as low as with pineapple juice. And I'm sure you could probably use lemon juice if you dilute it with water, but knowing how much water to dilute it with is hard to say without a way to measure pH.
Pineapple juice is perfect because it can be used straight from the can, it gives you the right pH, and it comes in those little cans so you don't have to buy more than you need.
Yeah. Peter Reinhart’s method started with six ounces of water, and the small cans of pineapple juice are exactly six ounces. Flour is the other factor. Pineapple juice has a low enough pH that when combined with flour, which acts as a buffer1, is still low enough to keep the pH under 4.5 after the first refreshment with water on day two, so it was sort of the magic combination.
I'd never heard it articulated before that it's possible that the yeast breaks dormancy because of a low pH and not simply because a specific pH is generally favorable to its proliferation. Have you confirmed that it really is a question of spore germination?
I have never come across any papers that have studied what it takes to activate wild sourdough yeast specifically. I don't think anybody is interested in spending research dollars studying it when there’s no money to be made or any health impact. The reference I have comes from a food microbiology textbook that says pH is a common spore activator (activation turns on the germination process). I think many people assume all it needs are fermentable sugars or oxygen or all these other nutrients thrown at it. But if that were the case, then yeast would start growing immediately, right? A fermentable sugar may be all that’s needed for germination, but germination follows activation—activation is like flipping the on switch. I like to think of it like a thermostat and a furnace. When the temperature drops to a certain setpoint, the thermostat kicks the furnace on, and only then does the furnace begin blowing warm air. The difference is that once the yeast are active, they stay active even when the pH rises. If you prefer, you can think of it like a game of Simon Says.
I can't tell you for sure that it’s the pH here, only that it sure appears that way, and as I said, it’s a common activator. If it's not pH, it's likely something else that is being produced by heterofermentative lactobacilli at the same time, in which case, pH is still a useful indicator. When the pH has dropped enough, yeast just come to life. That is the pattern I see again and again.
When did you start to think that maybe it wouldn't be necessary to use pineapple or another acid if you were to begin with a more concentrated source of yeast spores?
I never found that adding a yeast source works any differently (except bakers’ yeast which is already active), because even in the flower experiment the yeast didn't start growing and expanding the starter until the pH dropped. They activated on day three or four. All the gluten had disappeared, and the pH was less than 4.0 at that point. Even with flowers or any other plant material you might use, the bacteria come to life first, they move through their succession lowering the pH, and yeast join in at a later point. So basically I found it was exactly the same progression either way.
With pineapple juice, you're simply skipping the first of the bacterial phases. It isn’t necessary, but helps move the process along. With flowers or another plant source, you're going through the initial bacterial stage but moving through it faster because it’s providing a wider variety of bacteria—possibly some that are better suited for sourdough.
For the sake of Wordloaf readers, can you describe how that flower experiment worked and what you saw relative to your previous experiments?
The idea came from a 2016 paper that Michael Gänzle was kind enough to send me about using plant materials as the initial inoculum. The fascinating thing about it was that with several of the different materials that they trialed, they had Lactobacillus sanfranciscensis (currently named Fructilactobacillus sanfranciscensis) growing very early on, which is pretty much unheard of. In the simple flour-water pastes that had been studied until then, they never got F. sanfranciscensis before the cultures had been perpetuated for at least a few weeks. So it is really interesting that some of their plant-inoculated mixtures had F. sanfranciscensis growing from even the very first cycle.
Also, over the past 10 years I had been writing short installments in a series on where the microbes in sourdough come from. In doing the research for those, I found a theory in chapter 12 of the Handbook on Sourdough Biotechnology (Gobetti and Gänzle, Eds.) that fruit flies could be a reservoir for F. sanfranciscensis in nature. That resonated with me, because I know how attractive sourdough is to fruit flies—anybody who's ever left sourdough on the counter knows how attractive it is to fruit flies.
Before Dr. Gänzle sent the article, one of the things I asked about was whether the fruit fly connection had panned out. He said no, it didn't turn out to be the smoking gun, but that there did seem to be an insect connection. Many of the plant materials they used were flowers or fruit—structures that are visited by insects for pollen or nectar.
That was what led me to go out into my own yard and start collecting flowers and things. I didn't want to use fruit other than berries, because one of the things noted in the paper was that there was a higher correlation between acetic acid bacteria and cultures that had been started from apples or apple blossoms. And I know that sounds really attractive to people who think they want more acetic acid in their sourdoughs, but too much acetic acid is known to have a negative effect on yeast.
The Rob Dunn lab had done a series of tests on the different cultures that they collected from home bakers (and a few from professional bakeries) to see how fast and how strongly they each rise. The weakest risers were the ones with the acetic acid bacteria in them. Acetic acid is toxic to yeast in concentration. So for people who are doing all the right things but complaining about not being able to get their starters very robust, it could be that they've got some acetic acid bacteria in there that are just not letting those yeasts do what they are meant to do.
The first time I tried the process with flowers, my wild plum tree was in bloom. It really attracts insects, so I thought it was going to work great. This was following pandemic times when good flour had been hard to come by. I still had some off-brand white flour in my pantry that I wasn’t too happy with, and thought I would use it for this experiment since the paper seemed to indicate that the plant materials would provide all the microorganisms the starter needed. And it made sense there should be yeast there too. I'm sure there is, but for some reason my starter, even though it went through all the bacterial stages, never progressed to rising.
When I studied the procedure used in the paper, I realized it was kind of working against itself. It started with a mixture of 20% flowers, 100% flour, and 100% water. It was kept at room temperature and refreshed every 48 hours with 20% of the previous mixture. But from my own experience having made many, many cultures and walking so many people through the process, I knew that even if you skip a day, a refreshment of 20% into 100% each flour and water when yeast aren’t yet active is too big a refreshment. It dilutes all that beautiful acid that you're trying to build up. I think that's probably why it didn't take off, because not only was it flushing out the acid, it was flushing out the flowers quickly too. So I was losing my source of yeast at the same time as I was losing the acidity.
That was why I changed the procedure when I did it the second time.

This time around, rather than just using flowers from one source, I collected them from as many different plants as I could find. There were at least a dozen, maybe twenty different wildflowers and plant materials. And I changed the procedure by using whole-grain flour, because that would at least guarantee that I had some yeast in there to wake up since I still wasn't quite sure what was going on with my first trial. I decided to forego the feeding every other day and just stir it once a day. After 72 hours, the pH dropped enough that I felt it was safe to do a small feed. Following that, the pH dropped a little more, the yeast sprang to life, and it became a really wonderful starter.

I still have that starter today—It’s the best one I've ever made. It was really fragrant, very robust, and it made really good tasting bread. It was fascinating, the fragrances that I got from that original wildflower one. I had one or two small wild onion flowers in the mix, and I could really smell the oniony aroma until I finally diluted out all of the plant material. So it went through some really savory smells of ham and cured sausage before giving way to a floral scent when the flowers were cleared. The control starter without the flowers (just flour and water), had a nice orangey scent but the bread made from it didn’t have quite the same complexity as the flower-initiated one. It was a subtle difference, but discernible in the side-by-side tasing. I don't know that I've necessarily kept all the original bacteria that gave me that wonderful flavor and aroma. It stayed wonderful until I started storing it in the refrigerator for lengths of time, where I feel it may have lost some of that magic. It’s hard to find any floral aroma these days, but I sometimes get a whiff of the same orangey scent I smelled in the control and it still makes fine breads.

I did two more trials about a year later, both with single-source flowers. I collected flowers from the plum tree again, and that one went through some really amazing stone-fruit type smells. In the beginning it smelled like almond extract. Then it started to smell like cherries. And then in the end it smelled like mixed berries. I did another one with my hydrangea bush. I had trimmed off a bunch of wild growing branches that had flowers on them, so I used those. The initial mix on that one had a really, really perfume-y smell and by the end had a lovely honey scent. Interestingly, that hydrangea really attracts honey bees.
Every time I have used flowers to start these things I can tell there’s a different combination of microbes because they are what is producing these aromas after the plant materials have been diluted out. I could tell by the smells that I had something different each time.
But I think the yeast that I got in that second experiment was probably from the whole-grain flour rather than from any flowers because their rises were equal in time and height in both starters and doughs. By all outward appearances, the leavening element was the same. That inspired another experiment to see if there are yeasts on the flowers, and if there are, are they compatible with sourdough? So I made a yeast water with the flowers, which did get bubbly. Unfortunately, life intervened at that point, and I wasn't able to go ahead and turn it into a sponge or sourdough to see if it would raise a flour mixture.
I'm curious to know how many starters you have now. How do you choose one or another to bake with?
That's a good question. I don't usually keep all the starters. I will keep them until I'm done experimenting with them, and then they usually end up in the compost bin. I do still have a few in the refrigerator just because I was afraid to throw them out. I might want to go back and do some more work with the ones from the hydrangea and the plum tree. But I haven't had those out in over a year. So while I think they would probably still come back to life, there's no expectation that they'll have everything that they started out with.

But the one from the mixed wildflowers is still my favorite. I keep one from it at 100%-hydration and another that’s kept firm. The firm one I keep at 55% hydration ± 5% and generally try to feed it twice a day when it’s at room temp. I've been using that one to make panettone and it has been working fabulously. Even the 100%-hydration starter has made good panettone. Whether it's because it's got something special from the flowers that makes it well-suited, I couldn’t say. Probably not, but I really like that starter.
I choose between the two based on the bread I want to make. The firm one for mild, loftier breads, and the liquid one for breads that benefit from a little more acidity or enzyme activity. I keep even the liquid one fairly mild. And then there’s the practical consideration of which one is in the best shape and can be ready to go when I want it. Sometimes that makes the decision.
If someone was going to try the flower experiment, would you recommend not also using pineapple juice in the initial mix? I assume the answer is yes, to avoid potentially killing off the bacteria on the flowers, but I know people are going to wonder: If both are good methods, why not combine them?
That’s one of the experiments I have been wanting to try (in the queue with many others), but haven’t gotten around to it yet. You know, life. I think adding an acid would probably work great, but that’s only an educated guess until actually put to the test. I would love feedback from others who give any of this a try. None of the acids I recommend are strong enough to kill sourdough-compatible microorganisms when adjusted to a safe pH.
I recently watched Michael Gänzle’s On the Rise lecture on the science of sourdough starters. In it, he mentions that he found that F. sanfransicencis does not survive fridge temperatures. He also said that the way a starter is fed—whether it's held continuously at room temperature or moved in and out of the fridge and fed periodically—affects the ecology of what's dominant in it.
I'm wondering if you think that bacteria like F. sanfransicencis survive enough when stored in the fridge to revive when the culture gets treated the way it would like to be treated. Or if you think things like it die off under fridge conditions, never to be revived, in which case, whatever you get is something new after it is revived at room temperature.
Either scenario is possible because if a starter is kept out at room temperature and fed over and over, there is a chance that F. sanfransicencis could find its way back in at some point. Or if there are any still in there that are viable, it will probably come back when room temperature maintenance resumes and goes through enough cycles.
One study—I don't remember whether it was in the plant materials study or one of the other studies I’ve read—showed that it doesn't take much of a difference in the growth rates between species or strains—even as little as 10% higher—for the faster one to establish dominance pretty quickly. So if there's even a few F. sanfranciscensis in there they will generally grow faster than other species when conditions are favorable, and will have a tendency to rise to the top.
Ultimately I can't answer which will survive refrigerator storage and which will not, but it does seem like whatever initially gave my starter its floral scent didn’t do well in the refrigerator. If what I had was F. sanfranciscensis, I do think it was lost or damaged. How long it takes to kill them off, or whether they're eventually all killed off, I can't say. But there's always the chance that you can reintroduce them. If I went out at the same time of the year and collected all these wildflowers again and fed them back into my starter, perhaps there's a chance they could be reestablished. I haven’t tried that yet.
In that On the Rise lecture, Dr. Gänzle talked about three different types of starters. He called them bakery, farmer baker, and amateur or something pretty close to that, but I think continuous, intermittent, and occasional (or sporadic) would be more descriptive. The bakery starter is fed six to seven days a week at room temperature, not refrigerated. The farmer baker starter is stored in the refrigerator three or four days a week and taken out to prep for baking on the weekend as for farmers markets. The amateur starter is not used on a regular basis and spends most of its time in the refrigerator. Each one of those scenarios brings about a different typical microbial profile.
I bake a lot of sourdough in general, but normally only once or twice a week. While testing recipes for the book, I have been baking every other day or so, and as a result I have been keeping my starter at room temperature and feeding it twice a day. I’ve noticed a huge difference between this starter and previous ones that went in and out of the fridge.
Maybe the farmers market baker starter represents a happy medium between the two extremes? Because it is in the fridge for no more than a few days at a time, more activity is retained relative to one that is in cold storage for weeks or more. Many home bakers struggle to achieve a starter that behaves or performs the way they see other bakers’ starters do, and they'd probably benefit from knowing that there is an easy middle ground between continuous room-temperature maintenance and simply leaving it in the fridge all the time.
Yeah, a lot of people get hung up on thinking there's a “right” way to do it, or that it has to be all-or-nothing. My approach is much like yours, where I'll have it out at room temperature and feed it twice a day, until I'm basically done with it and need a break. Then I usually park it in the fridge, where it might be for a week. Ideally, when I’m not working with them, what I like to do is get one out on Friday night, feed it, feed it Saturday morning, Saturday night, Sunday morning, and then put it in the refrigerator Sunday afternoon. At some point in that scenario I might bake with it. The following weekend I’ll get out the other and do the same. But I find that refreshing at least three times in a row helps to get it back to where it was when it was put into the refrigerator. Having said that, the reality is that sometimes one will be in the refrigerator for months at a time. Like you, I’ve noticed that when I do work with a starter at room temperature for long periods of time—more than a month—it is more robust than when it's refrigerated 5 days a week and fed through the weekends.
Some people develop problems with their starter after they keep it in the refrigerator all week, maybe get it out on the weekend, feed it, and put it right back in the refrigerator without really allowing any time for it to have a full normal cycle. It's completely different when grown that way than when grown out on the countertop at room temperature for weeks at a time.

I don't know if you've ever tried this, but there have been times when I have experimented with—instead of putting it in the refrigerator when I can't feed it for a couple of days—just leaving it on the counter. It goes quiet and separates into liquid on top with flour sinking to the bottom and it looks, for all intents and purposes, like it's kind of going dead. But as soon as I feed it, it starts to bounce back. So it doesn't even really need to go back in the refrigerator if it's just gonna be a few days. It feels to me that it retains more of its character by just staying out on the counter than when I put it in the refrigerator. It smells better and it rises better. I use smell a lot as my guide, because smell is such an important part of taste. And I figure as long as my starter smells good, it's going to make good tasting bread.
Meaning it can influence the pH of a mixture without being strongly acidic or basic itself. Antacids such as Tums contain calcium carbonate, which has a buffering effect on stomach acids. ↩
wordloaf Newsletter
Join the newsletter to receive the latest updates in your inbox.