Lifestyles of the Enriched and Famous, Part Four
On enriched breads and their ingredients—Sugars
This is post three in a multi-part series on the many ingredients that go into enriched breads. (The first post, on eggs, is here, the second, on milk and other liquid dairy products, is here, and the third, on fats, is here.) Today is all about sugar and other sweeteners.
But first I wanted to share something I forgot to mention in relation to liquid dairy products and have since added to that post. It has to do with formulas that contain relatively small amounts of liquid other than eggs, such as brioche:
Brioche is usually made with high amounts of eggs and butter, and only small amounts of milk. In this case, I think it is fine (and simpler) to use water in place of milk. If your loaf only contains 50g of milk (for example), it’s only contributing about 5g of milk solids, which doesn’t amount to much. Leaving it out won’t make a noticeable difference, especially when you’ll have loads of milk solids coming from the butter instead. (Not to mention all the proteins provided by the eggs.)
On the other hand, if you use nonfat milk powder in your enriched breads, you can always “super-enrich” your formula with extra milk solids beyond which that small amount of milk would have contributed. You’ll of course need to increase the hydration to keep the dough texture consistent—a good starting point is an additional 1% water for every 1% nonfat dry milk powder used.
Okay, on to sugars. (Hit me up with any thoughts or questions on the above, preferably on that post.)
Sugar and sugar syrups
Sugar is added to doughs to add sweetness, increase browning on crusts, tenderize the crumb, and slow staling. Sugar syrups like maple syrup, malt syrup, honey, molasses, etc., do all this and add their distinctive flavors and colors to the bread as well.
Unlike eggs (or flour), table sugar is a simple ingredient. Like salt, it is a crystalline product made up of one molecule (sucrose). Sugar syrups used in baking are slightly more complex, since they are made up of mixtures of different sugars (mainly sucrose, fructose, and glucose), trace amounts of other compounds, and water; but in terms of sugar concentration they are nearly as "pure"—they are still mainly sugars. But how sugar and sugar syrups behave in doughs is pretty complex.
Sugar and water molecules
Each water molecule is an oxygen atom connected to two hydrogen atoms, like this: H-O-H. Sugars, meanwhile, are chains of carbon atoms arranged in rings, decorated with –OH bangles all along them; in chemistry lingo, these are known as “hydroxyl groups.” To a water molecule, a hydroxyl group looks identical to another water molecule, so it is happy to hang out alongside it. This is why sugars dissolve so readily in water—they are all “like” molecules, getting along with one another swimmingly.
But sugar molecules are considerably larger than water molecules. When sugar is added to a dough, they “replace” some of the water in and around the gluten network. As a result, their presence makes it harder for adjacent gluten strands to find one another and link up. This is why sugars have a “shortening” effect on a dough not unlike that produced by fats.
Sugars and delayed gelatinization
The structure of a baked loaf is completed when its starches gelatinize and firm up in the oven. But in order for a starch to gelatinize, it must first encounter water (i.e., hydrate). The presence of sugar in a dough limits the ability for starches to fully hydrate, which delays the onset of gelatinization during baking. This has two effects.
First, it allows the loaf to expand further before firming up. This is one reason that enriched breads don’t require steaming early in the bake—the sugars (and fats) they contain keep the dough soft, despite the rising temperature within.
And secondly, this also leaves the loaf softer after baking. When you delay the onset of gelatinization, fewer starches end up fully gelatinized at the end of the bake. As a result, the texture of the crust and crumb end up softer than it would otherwise be. (Consider the texture of a dough relative to that of the loaf it produces—one is obviously much softer than the other. Delaying starch gelatinization shifts the final texture of a loaf a little closer to that of the dough it was made from.)
Sugars and shelf life
Sugars in a bread can keep a loaf softer longer, because sugars are hygroscopic, meaning they attract and hold onto water strongly. As a result, the water in the loaf sticks around longer, increasing the longevity of its softness. Of all the common sugars, fructose is the most hygroscopic, so loaves containing sugars high in fructose—honey or agave—should have a longer shelf life than those made with an equivalent amount of table sugar.
Sugars and starch retrogradation
But the hygroscopic nature of sugars cuts both ways: It can also pull water out of the gelatinized starches, causing them to retrograde. When a gelatinized starch retrogrades, it becomes crystalline and hard; this is what happens when a bread stales—its starches lose the water they contain and recrystallize. (As I have said countless times before, stale bread seems dry, even though it still contains plenty of water, at least at first.)
All of which means that when a bread contains high concentrations of sugar it can firm up more quickly than it would otherwise. The Armenian Easter bread choreg typically contains 20-30% sugar and is notoriously quick to turn rock hard, even with the high quantity of butter it also contains. This is the main reason I always add a flour scald to my enriched breads—to give them extra longevity, despite their high sugar content. (The scald acts as a moisture “sink,” keeping the starches and sugars surrounded with extra water, slowing retrogradation.)
Sugars, dough texture, and hydration
Granulated sugar contains practically no water, and is “dry” like flour, so you might expect that adding it to a dough without adjustments would result in a drier-textured dough. Sugar syrups contain between 17% and 32% water, so similarly you might think that adding one of them to a dough would increase the hydration of a dough—and soften its texture—only by as much water as it contains.
But, in fact, as far as a dough is concerned, those abundant hydroxyl groups on sugars make them behave just like water: they have a “liquifying” effect, making it looser and wetter than it would be otherwise. Maybe even more so, since the sugars are too large to “hydrate” the flour, so they remain unbound, potentially making the dough slightly more liquid than an equivalent amount of water would.
All of which means that you need to be conservative with hydration when sugars are involved. The simplest approach here is to pretend that the sugar—or sugar syrup—is water: if you are increasing or decreasing the amount of sugar/syrup in a formula, add or subtract an equivalent amount of the water in it (or even a little less or more, to be safe).
Sugars, browning, and internal temperatures
Since sugars in doughs promote browning, it’s especially important to be careful with your oven temperatures, lest the loaf burn. This is one reason most enriched breads are baked at moderate temperatures compared to lean ones—somewhere between 300˚ and 400˚F in most cases.
Another reason to bake enriched breads at gentle temperatures is to ensure enough of its starches have firmed up on the inside by the end of the bake. The delayed gelatinization produced by sugars helps leave the crumb soft, but you need to be sure enough of the starches are set to prevent the loaf from keyholing on cooling.
As I’ve mentioned before, I generally do not take internal temperatures of my breads, at least once I know the formula: I bake to color (dark), and in most cases this is a reliable way to ensure the loaf is set on the inside. But because of both the delayed gelatinization of starches and faster browning produced by sugars, I sometimes do give my enriched loaves a poke with a thermometer, just to be sure.
But: The delayed gelatinization caused by sugars means that even if the loaf has reached ~210˚F (99˚C) on the inside, it might still not be sufficiently set on the inside. I’ve found that some loaves need an additional 10 or 15 minutes after they’ve come to temperature, just to be sure they don’t keyhole. So the internal temperature is a useful metric, but not as an endpoint.
My baking setup and method
My current MO for baking enriched loaves is to start high, then drop the temperature and bake long: 400˚F (205˚C) for 10 minutes, and then 40–45 minutes at 325˚F (165˚C). (With buns and other low-profile things, I’ll bake for more like 20-30 minutes after the temperature drop.) I find that a hot start helps give maximum oven spring and dry out the crust inside the pan fully.
I bake enriched loaves slightly lower in the oven than I do lean breads, usually on the lower-middle rack, to slow browning on the top crust, and to increase the heat coming from below.
I either bake the loaves directly on the oven rack or on a thin baking steel, for a bit more oomph on the underside. I do not recommend baking on a heavy steel, since those are far too conductive. I also don’t think it is necessary to waste energy saturating a baking stone for enriched breads; if you don’t have a B&T-style thin steel, just set the pans on your oven rack.
When I drop the temperature to 325˚F (165˚C), I will set a timer for 30 minutes. When the timer goes off, I’ll look at the color of the loaf, and maybe take its internal temperature. If it is dark enough and/or close to 210˚F (99˚C), I’ll let it go for another 10–15 minutes. If not, I’ll give it 5 more minutes and check again.
Once I’m confident the loaves are set, I’ll immediately remove them from their pans to a rack. If they look at all pale or soft on the bottom and side crusts, I’ll return them to the oven on a sheet pan, usually on their sides (for a little extra support), checking their color every 5 minutes or so. Once the sidewalls are good and dark, the risk of keyholing is minimal, so that’s when I’ll remove them from the oven.
Okay, that’s it for enriched breads for now. What are your questions or comments on the inclusion of sugars in breads. And what did I miss on any of this?
—Andrew



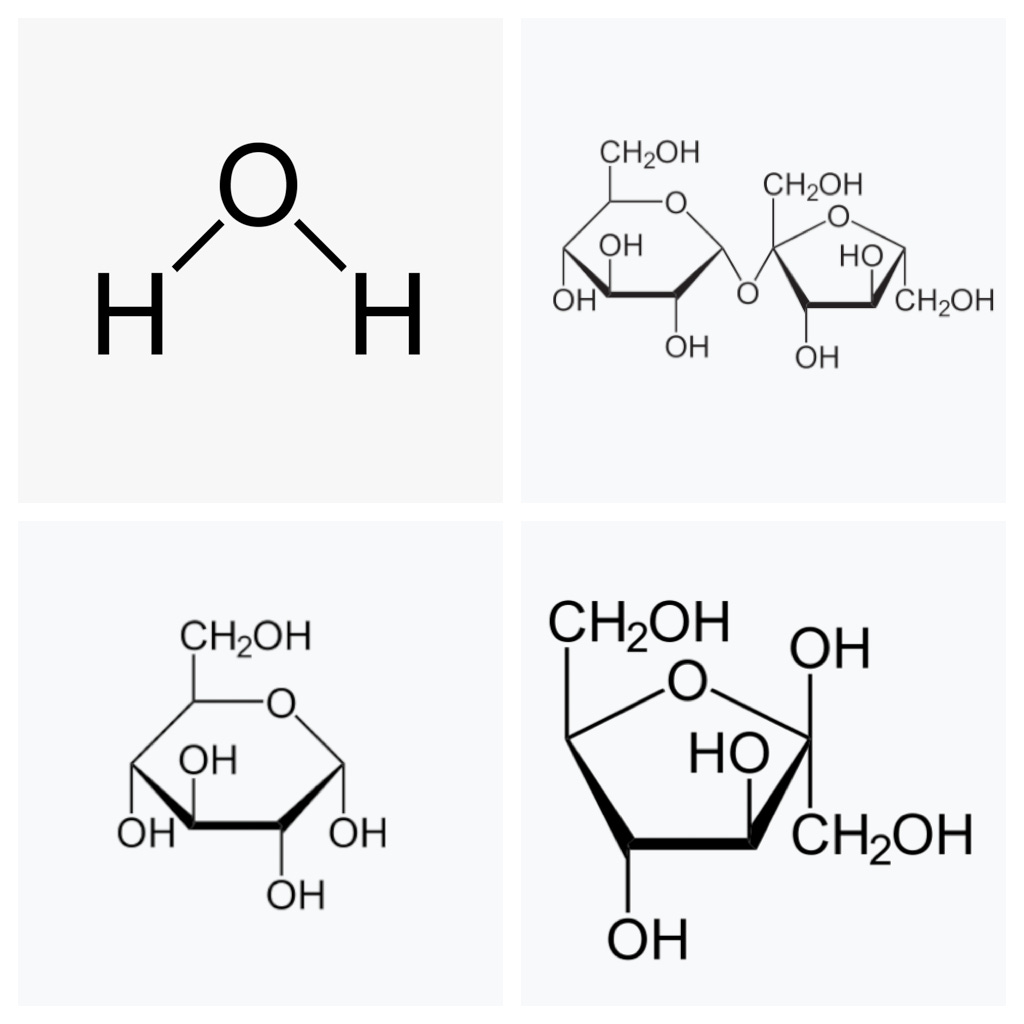

From a chemist—nicely done communicating sugar chemistry for non-chemists!!! Gold star for you!!!!
Thanks Andrew, these have been great posts on enriched breads. The second paragraph from the end doesn't seem right- "dark enough and/or close to 210...another 10-15 minutes... if not, 5 more minutes. Maybe I'm just reading it wrong.